
Headquarters
Fremont, CA, USA
Established
2008
Primary Industry
Medical Device, Medical Monitoring, Health Diagnostics, Healthcare Technology
Key Service
Research, manufacture, and sale of coagulation IVD products for professionals and self testers
Our History
Welcome to CoaguSense, Inc., where our commitment to improving the quality of life for individuals with coagulation disorders drives everything we do.
Our Story
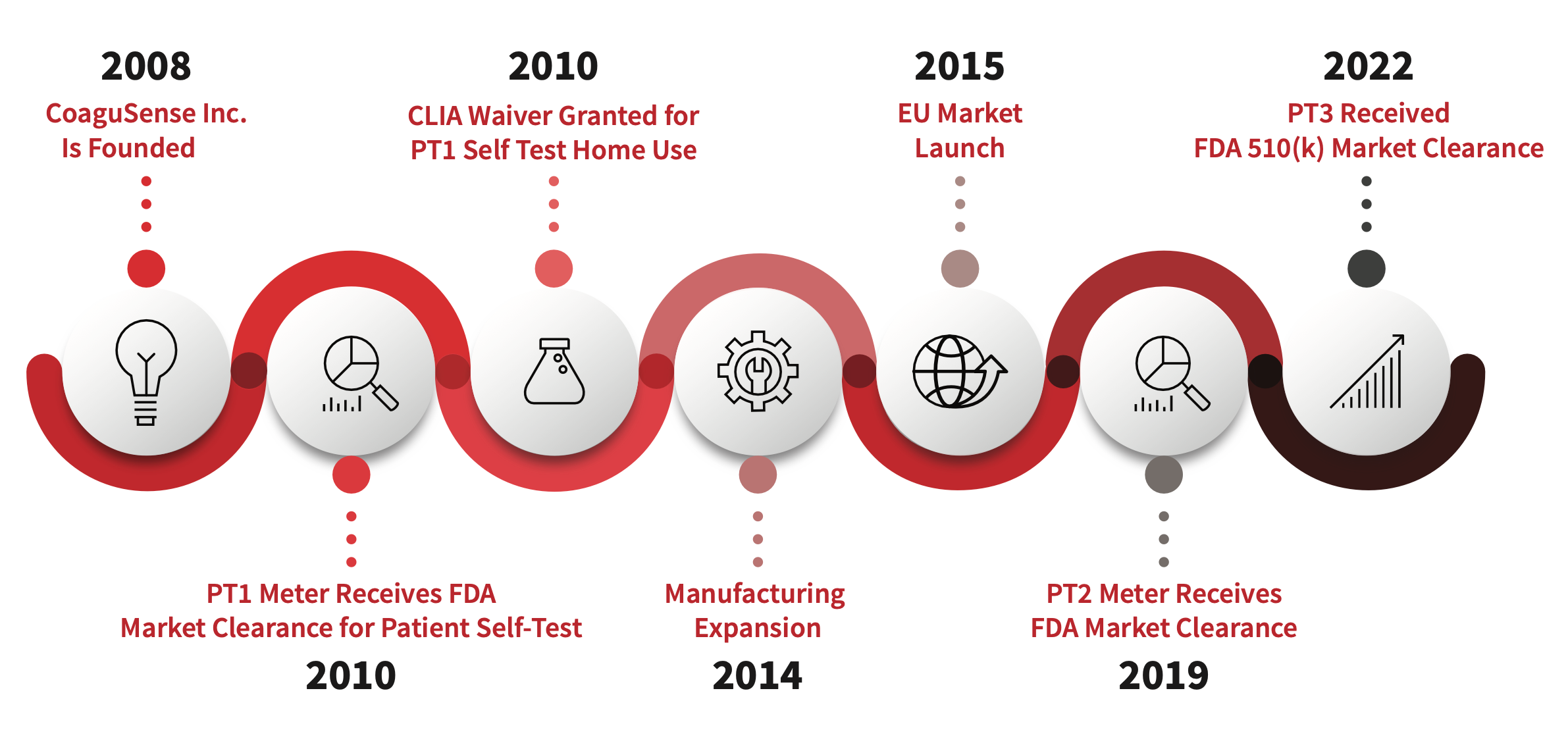
 CoaguSense, Inc., headquartered in Fremont, CA, has been pioneering advancements in coagulation disorder management since its inception in 2008. In 2010, CoaguSense introduced a groundbreaking Coag-Sense® PT/INR Monitoring System, the first of its kind to receive FDA market clearance for home use and a CLIA-waiver, allowing patients with conditions like Atrial Fibrillation to monitor their PT/INR levels independently.
CoaguSense, Inc., headquartered in Fremont, CA, has been pioneering advancements in coagulation disorder management since its inception in 2008. In 2010, CoaguSense introduced a groundbreaking Coag-Sense® PT/INR Monitoring System, the first of its kind to receive FDA market clearance for home use and a CLIA-waiver, allowing patients with conditions like Atrial Fibrillation to monitor their PT/INR levels independently.  Building upon this success, CoaguSense has expanded manufacturing capabilities since then at its Fremont facility, ensuring robust production standards and maintaining supply continuity, even during the challenges posed by the Covid-19 pandemic. This dedication underscored CoaguSense’s commitment to reliability and customer satisfaction.
Building upon this success, CoaguSense has expanded manufacturing capabilities since then at its Fremont facility, ensuring robust production standards and maintaining supply continuity, even during the challenges posed by the Covid-19 pandemic. This dedication underscored CoaguSense’s commitment to reliability and customer satisfaction.
The pinnacle of CoaguSense’s innovation came in 2019 with the launch of the Coag-Sense® PT/INR Monitoring System, also known as PT2. This second-generation device, cleared by the FDA for both professional and self-testing, sets a new standard in accuracy and affordability. Utilizing our patented Direct Clot Technology, the PT2 provides precise clotting time results in less than a minute, without the need for complex algorithms or look-up tables. Its compact design and versatile connectivity options enable seamless integration into hospitals, clinics, and homes, facilitating comprehensive anticoagulation management.
 Since its introduction in 2019, tens of thousands of Coag-Sense® PT2 meters have been distributed, underscoring the widespread adoption of CoaguSense’s cutting-edge technologies. This momentum continued in 2022 when we received FDA clearance for the Coag-Sense® Self-Care™ Monitoring System, the third-generation device in CoaguSense’s portfolio. Designed for simplicity and reliability, this system empowers patients to take an active role in their health management, ensuring consistent and accurate PT/INR testing from the comfort of home.
Since its introduction in 2019, tens of thousands of Coag-Sense® PT2 meters have been distributed, underscoring the widespread adoption of CoaguSense’s cutting-edge technologies. This momentum continued in 2022 when we received FDA clearance for the Coag-Sense® Self-Care™ Monitoring System, the third-generation device in CoaguSense’s portfolio. Designed for simplicity and reliability, this system empowers patients to take an active role in their health management, ensuring consistent and accurate PT/INR testing from the comfort of home.
Committed to excellence, CoaguSense is certified under rigorous quality standards including ISO 13485:2016 and Medical Device Single Audit Program (MDSAP) which comply with US FDA and EU In Vitro Diagnostic Regulations (IVDR). Looking forward, CoaguSense remains steadfast in its mission to innovate, improve patient outcomes, and streamline healthcare delivery globally. With a focus on expanding its reach and making life-changing technologies accessible to more individuals worldwide, CoaguSense continues to lead the charge in revolutionizing coagulation disorder management through dedication, innovation, and patient-centered care.
Locations
CoaguSense, Inc. is physically located in an industrial park in Fremont, CA near several other biotech, medical device, and technology start-ups. With less than 100 employees, we have a dedicated service-oriented staff. We answer the phone and help you improve your testing technique and troubleshoot your technical issues.
Headquarters
48389 Fremont Blvd., STE 106
Fremont, CA 94538, US
R&D
48377 Fremont Blvd., STE 111
Fremont, CA 94538, US
Manufacturing
48377 Fremont Blvd., STE 113
Fremont, CA 94538, US
